be2 bond order
One of the theories used to explain molecular bonding is Molecular Orbital Theory. The bond order for Be 2 is 0 zero.
 |
| Solved Draw An Mo Energy Diagram And Predict The Bond Order Of Be2 And Be2 Do You Expect These Molecules To Exist In The Gas Phase |
Your question is wrong because be2 molecule.
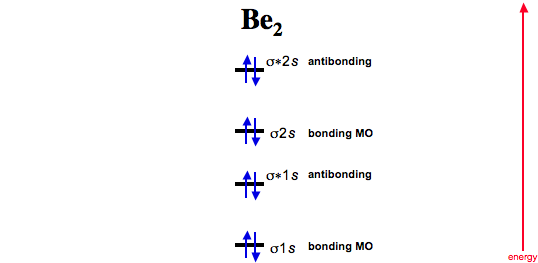
. Match the items in the left column to the appropriate blanks in the. O C -- O--- Be2-- O- -- C O _____ with one single bond between both Carbon atoms forming a 5-ring could not be drawn in this text. Be 28σ 1s 2σ 1s 2 σ 2s 2σ 2s 2Bond Order BO of Be 2 21440. For a bond to be stable the bond order must be a positive value.
Rm N_ rm b rm N_ rm a right frac 1 2left 4 4 right 0 ie no bond is formed between two beryllium. Be2 has a bond order of 05 and is slightly lower in energy than isolated Be atoms. Bond Order BO of Be 2 21440 Solve any question of Chemical Bonding and Molecular Structure with- Patterns of problems Was this answer helpful. The electron configuration of dihelium.
If the molecule He2 were to exist the 4s electrons would have to fully occupy both. Calculating the total number of electrons in He 2 Number of electrons in Helium 2 2 Number of electrons in. Likewise people ask what is the bond order for be2. See full answer below.
Because each beryllium atoms valence shell is. The valence shell of each beryllium atom is 2 s2 so there are a total of four valence shell electrons for which we. 0 0 Similar questions Which. After all what is be2s bond order.
Do you expect these molecules to exist in the gas phase. Predict the bond order of Be2 and Be2. 5 of bonding electrons of. Bond order frac 1 2left.
This theory deals with the generation of bonding and. Hence the bond order for He 2 is 05. We we draw the molecular orbital diagram for neutral di-beryllium we find a bond order of zero since there are four bonding electrons and four anti-bonding electrons. So the bond order of B2 is equal to 1 which you can get by drawing the molecular orbital diagram and performing the equation Bond Order.
Be2s bond order is 0 zero. The bond order for Be2 is 0 zero.
 |
| Shorter Is Higher The Strange Case Of Diberyllium Henry Rzepa S Blog |
 |
| Calculate The Bond Orders For Li2 And Be2 Molecules Using The Molecular Orbital Diagrams Given In Fig Brainly In |
 |
| Bond Order Of Be2 Is A 1 B 2 C 3 D 0 Class 11 Chemistry Cbse |
 |
| Use Mo Diagrams And The Bond Orders You Obtain From Them To Quizlet |

Posting Komentar untuk "be2 bond order"